



Ribo's proprietary technologies including unique delivery technologies for delivering oligonucleotide therapeutics for various targets and indications, as well as chemical modification technologies, novel , impurity analytical technologies, bioanalytical technologies, as well as manufacture technologies are comprehensively protected by patent, patent applications in major global pharmaceutical markets including China, the United States, Europe, Japan, South Korea and other countries/regions as well as trade secrets. Additionally, more and more collaborations with other companies in the research, development, transfer, license-out or license-in of patented technologies of pharmaceuticals and small nucleic acids are established and expected to advance the development of small nucleic acid pharmaceuticals. Ribo's IP portfolios and the collaborations with other companies provide solid support for the product pipelines of the company and enable us to possibly contribute to the health of mankind by meeting the unmet medical needs.
Recently two important platform technologies independently developed by Ribo have been granted patents in major jurisdictions, which relate to two key platform technologies for siRNA pharmaceuticals: RIBO-GalSTARTMdelivery technology and RSC2.0 siRNA chemical modification technology. Up to now, RIBO-GalSTARTM delivery platform technology has been granted patents or received Notice of Grant in China, United States, Korea, India, Russia, Australia, South Africa, HongKong, Macau and Taiwan. RSC2.0 siRNA chemical modification technology has been granted patents or received Notice of Grant in United States, Australia, Canada and Taiwan. It is expected that these two platform technologies will be granted patents subsequently in other jurisdictions.
Regarding siRNA delivery and chemical modification technologies, in the review article titled "Delivery of therapeutic small interfering RNA: The Current Patent-Based landscape" (Molecular Therapy: Nucleic Acids Vol. 29 Sep. 2022), Yu Chen et al. from University of Macau summarized 11,509 publicly available siRNA delivery technology patents worldwide from 2000 to 2020. According to the review article, the Chinese Academy of Sciences and Ribo are the only two Chinese entities in the TOP 20 list. Excluding universities and research institutions, Ribo ranks 6th among all biotechnology and pharmaceutical companies globally in the siRNA delivery patent list as shown in the table below.
As of September 25th, 2024, RIBO has been the patentee of 120 patent families worldwide, totaling 378 applications and patents among which 180 patents have received Notice of Grant or been granted. These two important technologies being grant patents recently fully reflects RIBO's research and development innovation capabilities and comprehensive intellectual property protection system. Ribo will continue to carry out its patent layout strategy and protect key technologies globally.
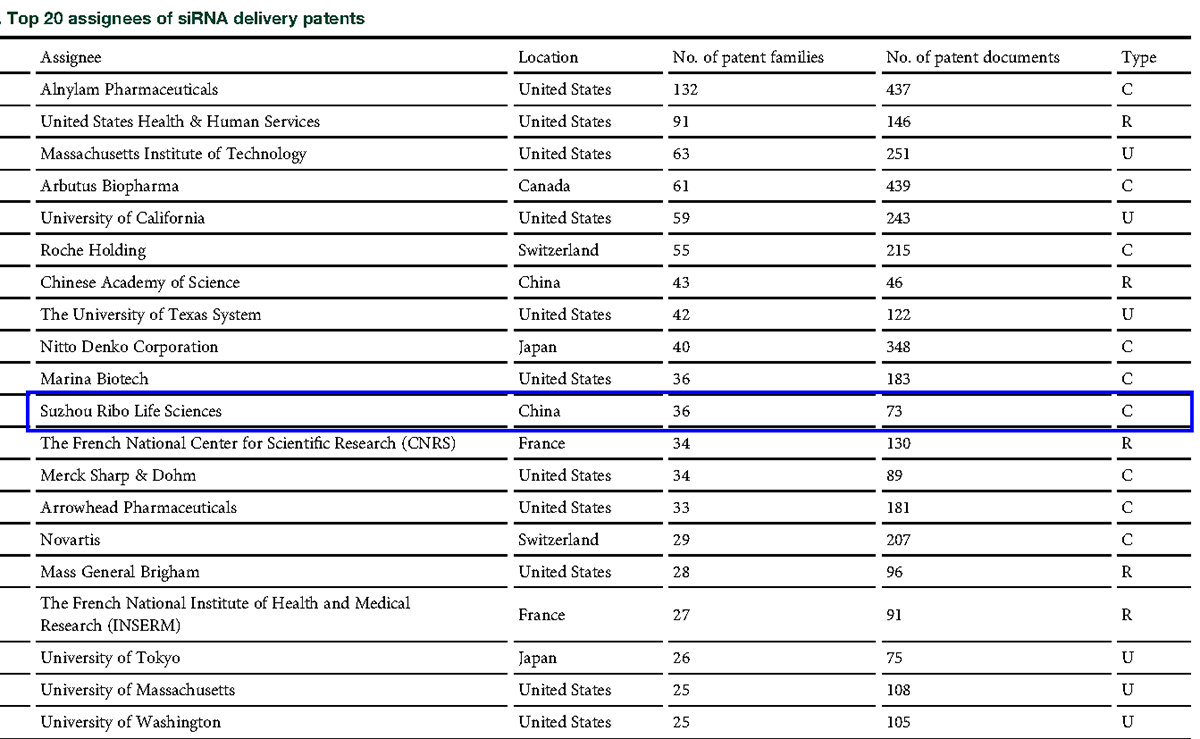
(Image from: Delivery of therapeutic small interfering RNA: The Current Patent-Based landscape. Molecular Therapy: Nucleic Acids Vol.29 Sep.2022)